Are you having trouble finding 'protons neutrons and electrons homework answers'? You will find the answers here.
Table of contents
- Protons neutrons and electrons homework answers in 2021
- Protons neutrons and electrons practice worksheet pdf
- Protons, neutrons, and electrons practice worksheet quizlet
- Worksheet protons neutrons and electrons
- Protons, neutrons, and electrons practice worksheet doc
- Protons neutrons electrons atomic # and mass # worksheet answers
- Atoms protons neutrons electrons worksheet
- Protons neutrons and electrons practice
Protons neutrons and electrons homework answers in 2021
 This image demonstrates protons neutrons and electrons homework answers.
This image demonstrates protons neutrons and electrons homework answers.
Protons neutrons and electrons practice worksheet pdf
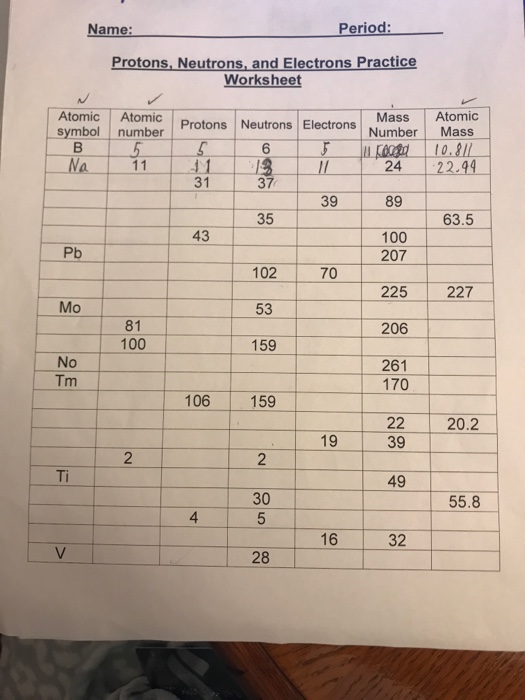 This image shows Protons neutrons and electrons practice worksheet pdf.
This image shows Protons neutrons and electrons practice worksheet pdf.
Protons, neutrons, and electrons practice worksheet quizlet
 This picture representes Protons, neutrons, and electrons practice worksheet quizlet.
This picture representes Protons, neutrons, and electrons practice worksheet quizlet.
Worksheet protons neutrons and electrons
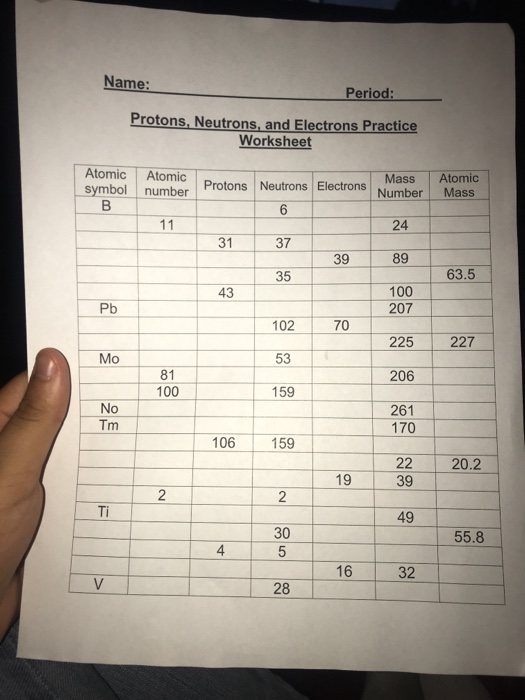 This image shows Worksheet protons neutrons and electrons.
This image shows Worksheet protons neutrons and electrons.
Protons, neutrons, and electrons practice worksheet doc
 This image shows Protons, neutrons, and electrons practice worksheet doc.
This image shows Protons, neutrons, and electrons practice worksheet doc.
Protons neutrons electrons atomic # and mass # worksheet answers
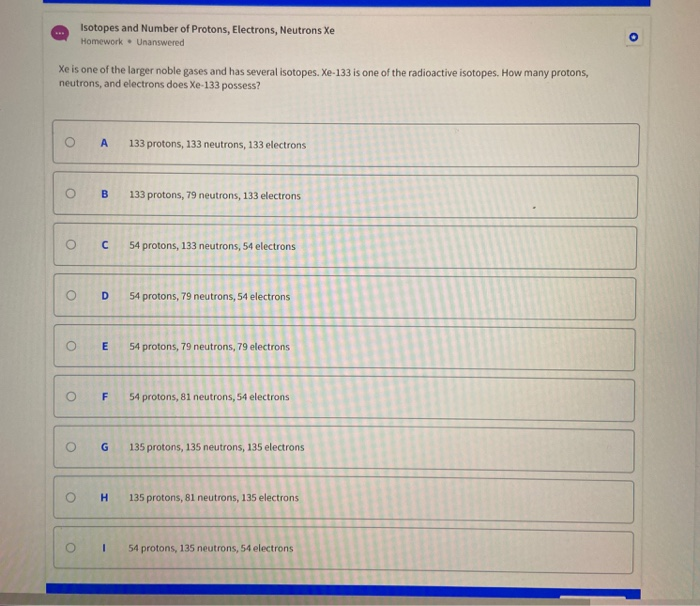 This image demonstrates Protons neutrons electrons atomic # and mass # worksheet answers.
This image demonstrates Protons neutrons electrons atomic # and mass # worksheet answers.
Atoms protons neutrons electrons worksheet
 This picture representes Atoms protons neutrons electrons worksheet.
This picture representes Atoms protons neutrons electrons worksheet.
Protons neutrons and electrons practice
 This image representes Protons neutrons and electrons practice.
This image representes Protons neutrons and electrons practice.
How are the number of protons in an atom set?
The higher the number of its protons, the bigger the nucleus is and the bigger the atom is. The variety of protons is set by the atomic number. The range of protons of an atom can’t change via any chemical reaction, and that means you add or subtract electrons to acquire the right charge.
Is the range of neutrons and protons the same?
Often, but not always, the amount of neutrons is the very same, too. The range of protons and neutrons could be equal for some elements but aren’t equal for all. The higher the number of its protons, the bigger the nucleus is and the bigger the atom is. The variety of protons is set by the atomic number.
Where are the neutrons located in an atom?
It is situated within the nucleus. The number of neutrons present in the nucleus of an atom is called the neutron number. Atomic mass or mass number is the total number of nucleons within the atomic nucleus.
How to calculate the number of electrons in a carbon atom?
So number of electrons in is also 6.The number of neutrons present in the carbon atom can be calculated as follows:Number of neutrons = Mass number (A) –Atomic number (Z)Substitute 13 for mass number and 6 for the atomic number in the above equation, we get: So the number of protons, electrons, and neutrons in are 6, 6, and 7 respectively.
Last Update: Oct 2021
Leave a reply
Comments
Breia
20.10.2021 04:58Protons neutrons and electrons practice worksheet. It is an ion of tritium isotope of hydrogen.
Cressida
20.10.2021 05:39To play this test, please finish redaction it. The nucleus contains the key construction blocks of life: the pairs of protons and neutrons.
Gumaro
23.10.2021 06:04• electrons surround the nucleus. The tiny particles called atoms ar the basic construction blocks of complete matter.